electron configuration for radon|unabbreviated electron configuration for barium : Baguio Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . Order Chocolates Online at Carrefour Qatar. Get the latest offers and shop from a large selection of Food Cupboard in Doha & Qatar. Great deals with up to 70% off. Free Delivery, Fast Shipping & Easy Returns!
PH0 · zr4+ electron configuration
PH1 · unabbreviated electron configuration for barium
PH2 · radon valence electrons
PH3 · radon on periodic table
PH4 · radon number of electrons
PH5 · full electron configuration of platinum
PH6 · full electron configuration
PH7 · element symbol for radon
PH8 · Iba pa
Pngtree’s contributor project supported 3 methods for cooperation. These 3 methods that depend on the review team to determined, you can accept it or not, after 1 day if you didn’t choose to approve or not approve the deal for the reviewed artworks, these artworks will automatically be shown on Pngtree. If you have any doubt for the deal .
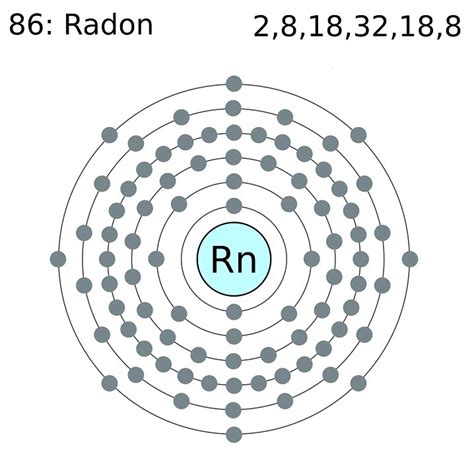
electron configuration for radon*******The electron configuration of radon is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p6, if the electron arrangement is through orbitals.
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .electron configuration for radon A step-by-step description of how to write the electron configuration for Radon (Rn). In order to write the Rn electron configuration we first need to know the . Radon condensed electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6. It is the same as the ground state .Radon. (Rn) Radon is a chemical element of the periodic table with chemical symbol Rn and atomic number 86 with an atomic weight of 222 u and is classed as noble gas and is .
Fluorine Electron Configuration. Neon Electron Configuration. The radon is also the immediate decay product of radium. Its most stable 222 Rn, isotope, has a half-life of only 3.8 days which .Atomic Structure of Radon. Atomic Radius: 1.34 Å. Atomic Volume: 50.5cm 3 / mol. Covalent Radius: Cross Section (Thermal Neutron Capture) σ a / barns: 0.72. Crystal .
Electrons & Oxidation. Nuclear. Isotopes. Mass Number. The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum of the number of .Characteristics: Radon is one of the noble gases; hence it is a chemically inert, monatomic gas. It is also radioactive, colorless and odorless. Radon is produced naturally by the decay of uranium’s decay products, such .

Radon has been used in implantable seeds, made of gold or glass, primarily used to treat cancers. Radon - Properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, .
electron configuration for radon unabbreviated electron configuration for bariumRadon has been used in implantable seeds, made of gold or glass, primarily used to treat cancers. Radon - Properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, . Beyond these direct applications, we note that since the advent of the periodic table, arranging systems by their nuclear charge proved sensiblepettifor_1984 .Not just from the point of quantum alchemy, but computational chemistry as well, classifying systems by their electron number might be a fruitful concept, as proven by the content .
unabbreviated electron configuration for bariumMercury has the electronic configuration of [Xe] 4f(14) 5d(10) 6s(2). So you can see that like Beryllium, it is also have a completely filled s orbital. Also an atom is more stable if the orbitals are half filled or completely filled. So it is reluctant to loose the electron. . and sends something like Radon, which even though it's Noble Gas .Nuclear structure of the odd-neutron radon isotopes radon-203,205,207
In the Periodic Table , The elements of group 18 are all gases, but there are highly unreactive. That’s why, they are called Noble Gases. The cause of there inertness is: Electronic Configuration. Every Noble Gas has completed its octet, means it has 8 electrons in its valence shell. Why are the elements in family 18 called inert gasses? In this work, we present a general spin restricted open-shell Hartree–Fock (ROHF) implementation that is able to generate self-consistent field (SCF) wave functions for an arbitrary configuration state function (CSF). These CSFs can contain an arbitrary number of unpaired electrons in arbitrary spin-couplings. The resulting method is . 1. Introduction. Natural radon isotopes can be used as tracers of various chemical and physical processes in the environment. Because the half-lives of 219 Rn (t 1/2 = 3.96 s) and 220 Rn (t 1/2 = 55.6 s) are so short, it is difficult to measure them precisely. However, 222 Rn (t 1/2 = 3.83 d) is often measured and has been widely employed for .X-ray computed tomography (CT) has been extensively applied in industrial non-destructive testing (NDT). However, in practical applications, the X-ray beam polychromaticity often results in beam hardening problems for image reconstruction. The beam hardening artifacts, which manifested as cupping, streaks and flares, not only debase the image quality, but .
Hotels Near Quezon City Hall In Quezon City Review. 4.1/5 5 Reviews. RedDoorz @ Winter Hotel Araneta. Quezon City | 4.24km from city center. G. Guest User. 3.7 / 5. location is very convenient. just few steps away from Araneta shopping centers, SM, Coliseum, Bus Terminal and Cubao LRT..many shops along the way with cheap foods.
electron configuration for radon|unabbreviated electron configuration for barium